ApplePhenon® Apple Polyphenols
Origin:
Green Apple
Malus pumila Mill
Standards:
60% procyanidins powder
(UV)
Fields of Action:
Anti-Fatigue | Anti-Oxidant | Health Support for Skin, Weight Management
FROM THE LAND OF THE ORIGINAL APPLE
ApplePhenon® is carefully extracted from specially selected immature green apples. Using a proprietary gentle extraction process, ApplePhenon® from BGG preserves the highest concentration of apple polyphenols, strong antioxidant activity and an optimized profile of proanthocyanidins. Clinical studies have shown that ApplePhenon® plays an effective role in antioxidant, weight, glucose management, dental care, cardiovascular protection, athletic enhancement and allergy support. ApplePhenon® has been GRAS self-affirmed in USA and can be used in all food, beverage and dietary supplement applications.
- Patented: Protected by 5 international product, process and use patents
• Trademarked: Worldwide trademark registration
• Researched: Supported by more than 50 publications
• Tested: Clinically validated for 7 different health benefits
• Safe: Self-affirmed GRAS in USA
• Water Soluble: Suitable for beverages, cosmetics and dental products
• Highly Stable: 36 month shelf life
ADVANCED PROPRIETARY PROCESS
ApplePhenon® by BGG is a patented, proprietary polyphenol extract produced from, unripe apples. Using a proprietary mild extraction process and advanced purification technology, ApplePhenon® is produced to preserve high concentrations of apple polyphenols, strong antioxidant properties and optimized profile of proanthocyanidins. The starting raw material for producing ApplePhenon® are unripe apples containing at least ten times the level of polyphenols found in ripe apples.
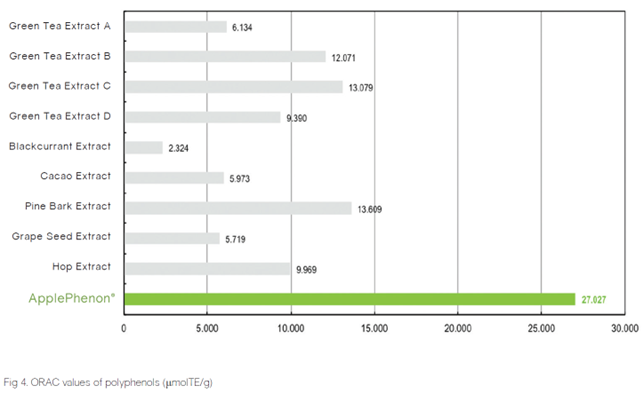
The ORAC (Oxygen Radical Absorbance Capacity) value of ApplePhenon® is extremely high in comparison to other polyphenol products.
WHAT IS APPLEPHENON®
Polyphenols extracted from unripe apples
UNIQUE PHYTOCHEMICAL BOUQUET
ApplePhenon’s active constituents are listed in the table below. ApplePhenon® possesses a unique phytochemical profile, with about 12% of flavanol monomers (catechin and epicatechin) and a high content of oligomeric proanthocyanidins.
ApplePhenon® has a much higher content of oligomeric proanthocyanidins compared to grape seed extracts. Green tea extracts contain monomer and dimers almost exclusively while grape seed extracts are rich in polymeric proanthocyanadins. This is of paramount import; in a pre-clinical trial, the polymeric procyanidins in ApplePhenon® positively and synergistically promoted the absorption of procyanidin oligomers (which are the only procyanidins that are absorbed by the human body).
HIGH BIOAVAILABILITY OF APPLEPHENON®
Oligomeric procyanidins in ApplePhenon® are more easily absorbed in blood than other polyphenol products.
Fig 3. Free non-conjugated procyanidins in rat plasma 2 hours after administration. A_ Free proanthocyanidins analyzed by Porter method (expressed as procyanidin B2) B_ Total amounts of procyanidins B1, B2 and C1 by HPLC/MS. Shoji, T.; Masumoto, S.; Moriichi, N.; et al. Agric. Food Chem. 2006, 54, 884-892.
NATURAL ANTIOXIDANT
According to the graph above, the ORAC (Oxygen Radical Absorbance Capacity) value of ApplePhenon® is extremely high in comparison to other polyphenol products.
CLINICALLY SUPPORTED BENEFITS of APPLEPHENON
WEIGHT MANAGEMENT
Pre-clinical studies demonstrated that ApplePhenon® can potentially decreases the transcription of genes involved in fatty acid synthesis similarly to a restricted food diet. It may also contribute to suppression of visceral adipose tissue accumulation.
Furthermore, is has been shown that administering apple polyphenols can potentially decreases the Firmicutes/Bacteroidetes ratio and increases the proportion of Akkermansia by a factor of eight. This influences the gut microbiota and the intestinal metabolome (which has beneficial effects on metabolic homeostasis).
Clinically, ApplePhenon® at 600 mg per day can play a role to be effective in decreasing visceral fat within an 8 to 12 week time frame in a randomized, double-blind and placebo controlled study in 94 subjects with BMI 25-30. In addition, in a smaller group of 30 subjects (BMI 18-30) an excessive dose of ApplePhenon® for 4 weeks has been shown to have no deleterious effects.
Similarly, the visceral fat area and the level of adiponectin in the Applephenon® group improved as compared to the control group in a 20 week (4 weeks observation, 12 weeks treatment, 4 weeks follow up) randomized, double-blind, placebo-controlled comparative study.
CARDIOVASCULAR SUPPORT
Several clinical studies demonstrated that Applephenon® plays a role in maintaining normal cholesterol levels in healthy subjects. In a 4-week pilot study with 48 healthy volunteers with cholesterol levels between 200 and 260 mg/dL, a dose-dependent decrease in total cholesterol and LDL cholesterol and an increase in HDL cholesterol were found at dosages of 300mg, 600mg and 1500mg per day [1]. These results were corroborated in a longer duration study with 71 moderately-overweight subjects (BMI 23 – 30 and total cholesterol averaging 220 mg/dL). Finally, in a small crossover study on 6 volunteers, ApplePhenon® at 600mg per day was shown to inhibit the absorption of triglycerides from a high-fat diet.
GLUCOSE MANAGEMENT
Recent preclinical studies suggest that chronic administration of ApplePhenon® help maintaining healthy blood sugar metabolism in obese diabetic ob/ob mice.
DENTAL CARE
ApplePhenon® can inhibit glucosyltransferase (GTE) of dental bacteria Streptococci mutans and Porphyromonas gingivalis, therefore preventing dental plaque formation. A clinical study on twenty 19 – 20 year old women who rinsed their mouths with 10 mL of ApplePhenon® solution (0.5 mg/mL) three to five times a day for three days showed a significant decrease of dental plaque formation. Chewing gum with a low dose of 0.024% ApplePhenon® for five minutes can inhibit the production of methanethiol (MeSH), the main cause of bad breath.1
SKIN CARE
Applephenon® has been evaluated in two clinical studies targeting the skin. In a first pilot study on 24 subjects it has been shown that oral administration of Applephenon® at 10mg/kg may be effective as a complimentary support for skin disorders. A second randomized, double-blind and placebo-controlled study on 59 healthy women at dosages of 300 mg and 600 mg per day over 12 weeks showed reduction in skin reddening (sunburn) and obvious skin-whitening effect versus placebo.2
RESPIRATORY SUPPORT
Studies have shown that ApplePhenon® can be supportive during allergic rhinitis challenges, as shown in a randomized, double-blind clinical trial on 33 patients with persistent allergic rhinitis. These patients were of various ages from 15 to 65 and had persistent moderate to severe symptoms for a period of at least three years. Patients in the treatment group were given a drink containing ApplePhenon at either a low-dose or high-dose level versus placebo. Results indicated that both the low-dose and high-dose treatment groups experienced significant improvements in sneezing attacks. In addition to improved sneezing attacks, the high-dose group also experienced reduced nasal discharge; and the percentage of people who showed improvement in the swelling of the nasal turbinate was higher in the treatment groups.
ATHLETIC PERFORMANCE IMPROVEMENT
In a clinical trial study on college athletes, 20 subjects (male and female) randomly took ApplePhenon® (1,200 mg/day), CoQ10 (300 mg/day) and placebo for 8 days (crossover). On day 9, performance was tested through a bicycle ergonomic exercise load measuring pedal speed differences between the first 30 minutes and the last 30 minutes. Compared with the CoQ10 group and the placebo group, the ApplePhenon® group experienced a significant improvement in performance levels.
For more information, please contact us.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Reference
-
Matsudaira, F.; Kitamura, T.; Yamada, H.; Fujimoto, I.; Arai, M.; Karube, H.; Yanagida, A. “Inhibitory effect of polyphenol extracted from immature apples on dental plaque formation” Journal of Dental Health 48, 230-235 (1998)
- Enomoto, T.; Nagasako-Akazome, Y.; Kanda, T.; Ikeda, M.; Dake, Y. “Clinical effects of apple polyphenols on persistent allergic rhinitis: a randomized double-blind placebo-controlled parallel arm study” J Investig Allergol Clin Immunol 2006, Vol. 16(5): 283-289
